Brain tumours are one of the hardest-to-treat cancers with a significant impact on the physical, cognitive, and psychological aspects of patients. Glioblastoma is the most aggressive type of brain tumour and few patients survive more than one year after diagnosis, with mortality rates unchanged over the last decades. Researchers are working on immunotherapy approaches to advance the outlook for patients with this aggressive cancer.

Common symptoms of brain cancer include headaches, seizures, memory problems and personality changes, which also inflict inevitably the patients’ families. Glioblastoma (GBM) is the most aggressive subtype of brain cancer, with a median survival of hardly one year after diagnosis. Treatments have not evolved over the last decades and so mortality rates remain unchanged, posing an urgent challenge that needs to be addressed.
Survival rates unchanged in 20 years
Current treatment routes for glioblastoma include surgical resection of the tumour, followed by radiotherapy and chemotherapy. Unfortunately, these treatments are not curative and the tumours quickly return, often more aggressive than the first time, for example with “tentacles” that spread all over the brain. Another reason for therapeutic failure is the leftover tumour cells remaining after surgery, since it is not possible to completely remove the tumour due to its very invasive and infiltrative nature. In addition, tumour cells’ show a high resistance to the offered radio- and chemotherapies. Life expectancy of GBM patients has not been improved significantly since 2005 and clinical trials with novel immunotherapies do not show promising results either.

“New therapies need to be tested in a preclinical setting first. The cancer models used in this phase must faithfully recapitulate patients’ reality so results can be reliably translated into the clinic. However, current available models suffer from the lack of immune system components, which makes it difficult to study the complete tumour microenvironment and limits the possibility of testing new immunotherapies. ”Pilar María Moreno Sánchez Biochemist and PhD candidate at the Luxembourg Institute of Health (LIH)
Patient-derived models a challenge
There is limited availability of GBM patients’ tissue, making the development of patient-derived models a challenging task. Moreover, GBM is also considered an immunologically “cold” tumour: the cancer does not induce a strong immune response, meaning identifying novel drugs that restimulate the immune system is key to improve survival rates for patients.
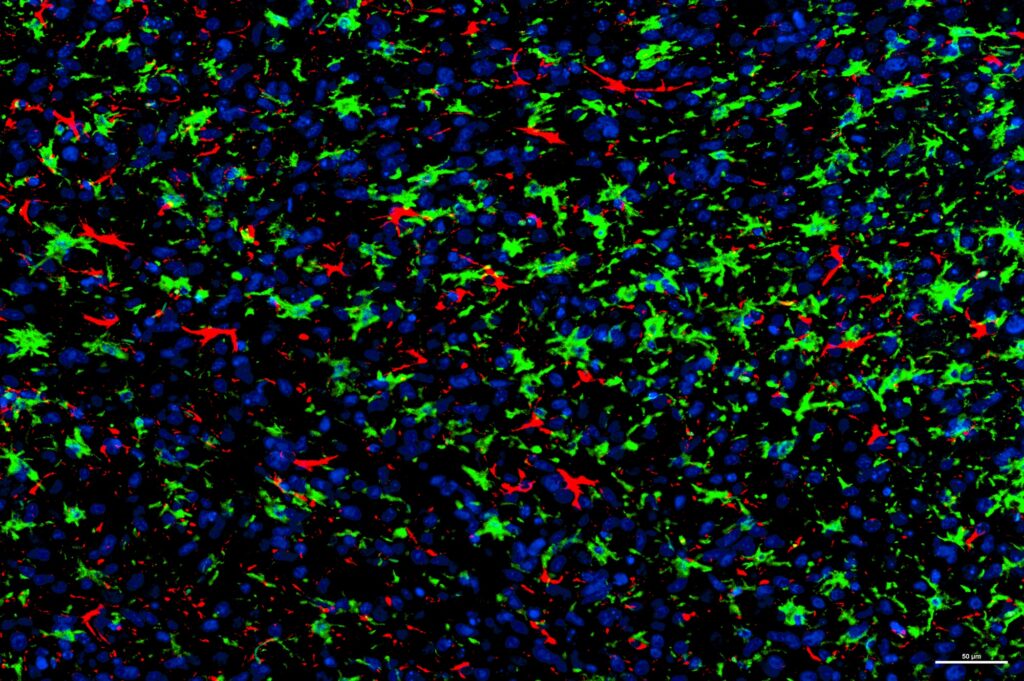

The NORLUX Neuro-Oncology laboratory at the Luxembourg Institute of Health has established a large cohort of GBM organoids and orthotopic xenografts derived from patient tumour tissue, used as patient ‘avatars’ in preclinical studies.
“In my PhD project, we use these models to study the impact of chemotherapy on the composition of tumour microenvironment and to understand how tumour microenvironment supports resistance to treatment.
“Moreover, we are expanding our cohort of patient avatars towards immunocompetent models by using so-called humanized mice, which have a partially reconstituted human immune system. Such models will provide a new window of opportunities for immunotherapeutic testing.”

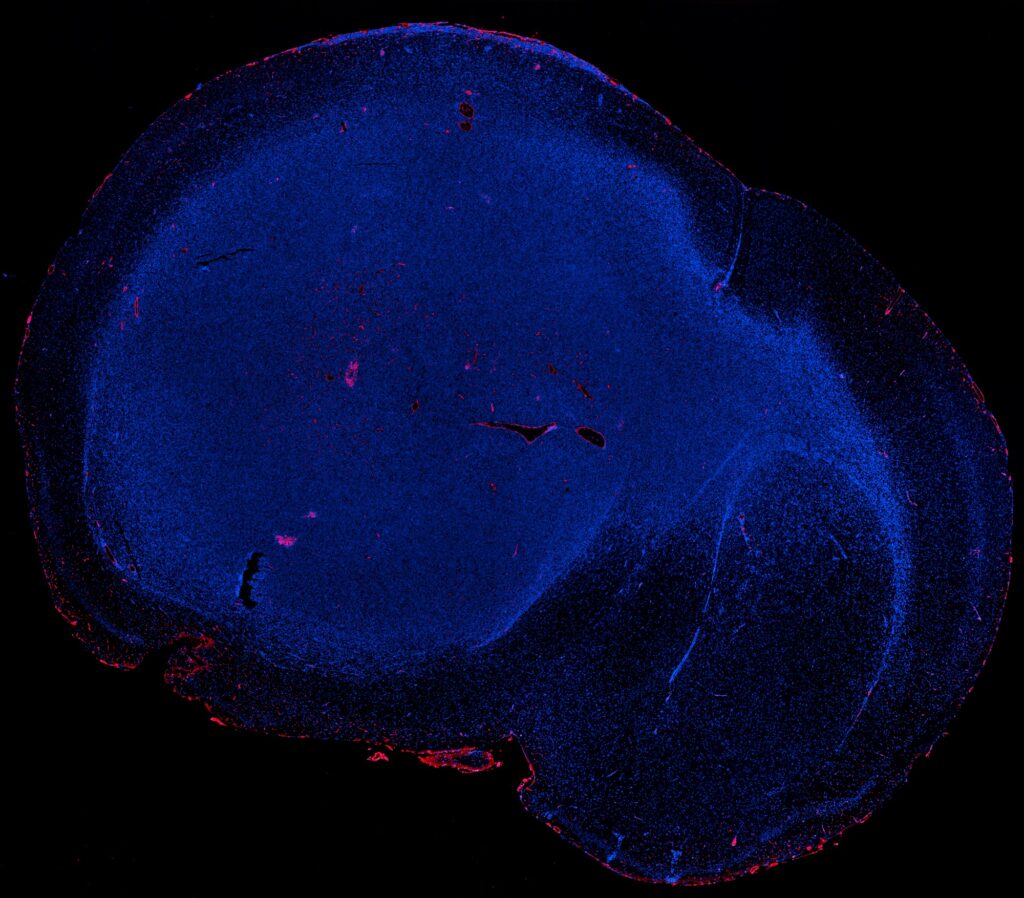
“We have been able to derive GBM patient-derived orthotopic xenografts in humanized mice and confirmed the infiltration of human immune cells into the brain of these models. These findings point towards the promising use of humanised mice as a tool for testing GBM immunotherapy.”
Pilar María Moreno Sánchez is a biochemist and PhD student at the Luxembourg Institute of Health in the FNR PRIDE Doctoral Training Unit (DTU) i2TRON.

MORE ABOUT PILAR MARIA SANCHEZ
Describing herself, the scientist
“I learned about cancer when I was 13 and my grandfather was diagnosed with colorectal carcinoma. I focused all my efforts towards pursuing a career where I could help other people in that situation and thus decided to study Biochemistry. The NORLUX group works in close contact with the clinics and thus it appeared as a good option to learn the steps of preclinical research.”
What she loves about science
“Probably the greatest part is knowing you can be the first one with a unique finding in your hands. But apart from that, research is a lifestyle where new questions are always coming up, which makes it an exciting journey full of curiosity and challenges.”
Mentors with an impact
“Yes, I fondly remember Fátima, my first mentor. She was my Organic Chemistry teacher at university who, very generously, guided and opened doors for me to get into the academic world of research. She helped me find my passion for science and was always there when I felt in doubt.”
Why she chose Luxembourg for her research
“The main reason was finding an adequate PhD fellowship since I knew I wanted to work on a project with a clear translational approach in Immuno-Oncology. I found the “Integrating immune strategies for Translational Research in Oncology and Neurology” (i2TRON) doctoral training unit and that was a very good fit for me. Additionally, Luxembourg is a very attractive country in terms of an international environment and is in a very convenient location for travelling, which is my biggest hobby.”
Where she sees herself in 5 years
“I plan to pursue my work in clinical research. I started with very basic research during my university studies, then switched to a more preclinical approach in my PhD and I would like to further get involved directly in clinical trials in my next career steps. That would make the perfect progress for me.”
Summarising her research in one sentence
“Advanced preclinical models to model GBM tumor microenvironment”
On her research, peer to peer
“The focus of this project is to expand NORLUX patient-derived orthotopic xenografts (PDOX) platform towards immunocompetent models for GBM immunotherapy. So far, we have investigated the crosstalk of the immune component with tumor cells based on different mouse strains and humanized mice, observing a clear functional crosstalk between the mouse-derived microenvironment and the human GBM cells in PDOXs. In particular, microglia, the resident immune cells of the brain, turned out as the subpopulation exhibiting a higher transcriptional adaptation, confirming a switch towards tumor-associated macrophages. Future functional assays will allow us to better understand the interaction between microglia and GBM cells.”
Related highlights
Spotlight on Young Researchers: Toward sustainable construction
The construction sector consumes over half of the world’s resources, contributes 11% to global CO2 emissions, and produces nearly 50%…
Read more
Spotlight on Young Researchers: Reconstructing the history of flowing waters
The hydrological cycle – the continuous circulation of water in the Earth-Atmosphere system – is vulnerable to climate change, which…
Read more
Spotlight on Young Researchers: Understanding self-assembly
Self-assembly is a spontaneous process where things naturally come together to form organised structures. This for example happens in our…
Read more
Spotlight on Young Researchers: Finding the optimal way to exploit plant metabolites
Plants synthesise a wide range of natural molecules, described as plant metabolites or phytochemicals with enormous industrial potential in anything…
Read more
Spotlight on Young Researchers: Designing tomorrow’s meeting rooms
Interactive wall-sized displays are beneficial for complex decision-making tasks such as visual data analytics, thanks to their large size and…
Read more
Spotlight on Young Researchers: Natural killer cell immunotherapy for a better outlook for pancreatic cancer patients
Pancreatic cancer is the 7th leading cause of cancer-related death worldwide, with more than 465.000 deaths reported in 2020 (WHO),…
Read more